Posted: January 28, 2023

Stability is a key issue when evaluating new phase-change materials (PCMs) or thermochemical materials (TCMs) for thermal energy storage. A loss of stability may reduce the storage capacity and/or the thermal power exchange over the operational life of compact thermal energy storage (CTES) systems. This is why the researchers of IEA SHC Task 67 Compact Thermal Energy Storage Materials have started to list relevant degradation factors with the aim of developing recommendations for stability testing based on simple experiments. We discussed the approach with two Task 67 experts, Christoph Rathgeber from the Bavarian Center for Applied Energy Research – ZAE Bayern in Germany and Ana Lazaro, Associate Professor at the i3a-University of Zaragoza, Spain. Their ultimate aim is to establish a definition of PCM and TCM stability.
Why is stability such a big issue for PCM and TCM materials?
Rathgeber: In CTES applications, PCMs and TCMs are continuously charged and discharged. PCMs are, for example, heated up and cooled down, thereby changing their phase – either from liquid to solid and vice versa or between two different solid states. In the case of TCMs, both heat and mass transfer take place. Many PCMs and TCMs are mixtures of different materials - blends or composite materials, e.g. combined with a solid matrix structure to improve their mechanical properties. Both the repeated heat and mass transfer processes and the complex nature of many PCMs and TCMs make investigations of their stability under application conditions an important field of R&D.
Lazaro: The stability of materials used for CTES is an important issue because the system should ideally maintain their performance as designed throughout the lifetime. A loss of stability may cause a loss of storage capacity and/or thermal power exchange over the operation time of the CTES.
Do you look at short-term stability (several cycles) or long-term stability (over the whole lifetime)?
Lazaro: The stability is important in both the short and long term, so R&D activities are focused on both time perspectives. Short-term studies could also provide information on the limits for the operation of the material, for example the maximum operating temperature for a certain material to ensure the stability or the required atmosphere to avoid moisture sorption, among others. Considering the long term is important to determine the lifetime of the system and also to investigate whether there are possibilities to recover the material to the initial state with the initial thermal performance.
Rathgeber: For many CTES materials, the charging/discharging behaviour within the first cycles is crucial. In the long term, stability testing under actual application conditions is needed to investigate the market readiness of CTES materials.
One of your objectives is to find out how degradation can be accelerated in tests in order to assess the stability of CTES materials more quickly. How does this work?
Lazaro: When a CTES is designed and a suitable storage material (PCM or TCM) is selected, the engineers would like to have as much information as possible on the stability of the material. Test routines for long-term stability take a long time, so any acceleration when testing stability will result on an increased number of materials whose stability has already been assessed.
Rathgeber: The idea is to provide experimental test routines with which the stability of a certain CTES material can be evaluated more quickly under given application conditions. For this purpose, it would be helpful to accelerate degradation processes in a controlled manner. The overall goal is to better understand the conditions under which degradation takes place. An improved understanding of degradation will help to select an appropriate CTES material for a particular application or to modify a CTES material so that it can be applied in a storage system with little or no degradation.
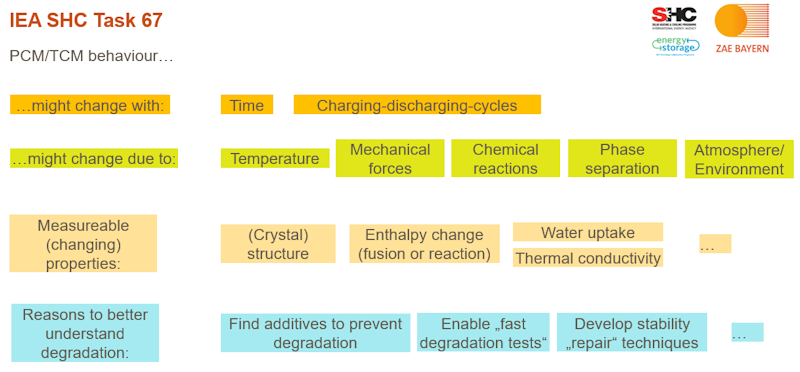
Template to assess factors which influence stability of PCMs and TCMs. It was drafted by the German institute ZAE Bayern and then discussed and extended during an IEA SHC Task 67 meeting. Scheme: ZAE Bayern
You have developed a template to map factors which influence the stability of storage materials in different colours. What goals are you pursuing with this template?
Rathgeber: The purpose of the template is, first, to collect the existing knowledge of the Task 67 participants on CTES material stability. Second, the template has been designed to structure the various aspects of stability and to support the discussion on CTES stability among the experts. As different CTES materials (material classes) exhibit different stability effects, the template is to be completed separately for different materials or material classes. Therefore, it will be distributed to the large group of Task experts who have expertise on specific CTES materials in order to complement it.
Lazaro: The template is also useful as a checklist of issues to be considered when selecting material and operation conditions during the design of a CTES system.
Wim van Helden, Co-Chair of Task 67, said in a meeting recently that “the aim is to develop a kind of 'cookbook'”. What does this mean here?
Rathgeber: As CTES material stability depends on the material (classes) and the conditions of a particular application, a step-by-step guide to testing and evaluating stability and selecting a suitable CTES material for an application could be a valuable outcome of our Task 67 subgroup. In addition, similar to a “cookbook”, we aim at providing recipes to improve CTES material stability based on lessons learned by the Task experts.
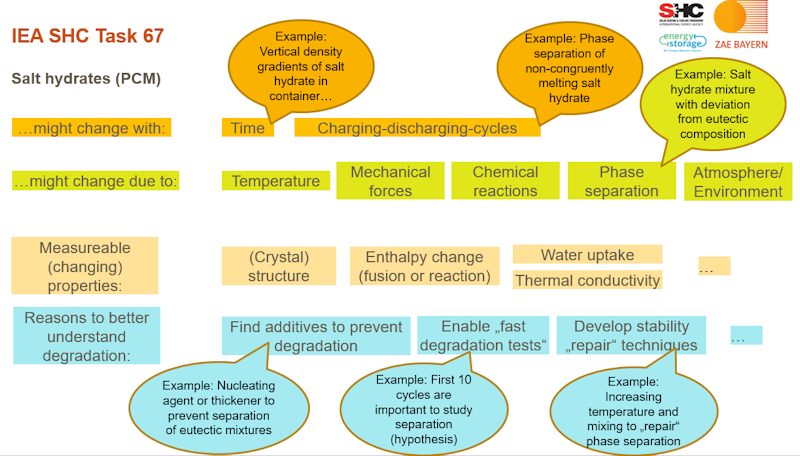
You have applied the template first to salt hydrates - a PCM material class. Can you further explain the blue bubbles, which include solutions to better understand the degradation?
Rathgeber: Salt hydrates are mixtures of salt(s) and water. During thermal cycling, some salt hydrates exhibit a separation of solid phases, e.g. different hydration levels of the salt. To counteract phase separation, thickeners can be applied to obtain a gel-like material in which the different solids are mechanically hindered from separating spatially. In some cases, phase separation of salt hydrate mixtures occurs if one of the mixture components supercools. To reduce supercooling, nucleating agents can be added to the mixture. The described phase separation of salt hydrates is often observed within the first thermal cycles (e.g. within the first 10 cycles). Thus, a testing routine that carefully examines the first thermal cycles of salt hydrate mixtures could be a way to evaluate their stability quickly.
Websites of organisations mentioned in this article: